It gives the following structure and has a multiple bonds and an adjacent atom with one lone pair of electrons. The second-row elements C N O F can only handle up to.
Solved Click The Draw Structure Button To Launch The Chegg Com
Lets draws many residents structures as possible for the following species.

. Two must-follow rules when drawing resonance structures. For a with H 3 cc double bond. Experts are tested by Chegg as specialists in their subject area.
This is the answer to Chapter 16. A CH 3 2 CHCH 2 CH 2 CHCH 3 2 b CH 3 3 CCH 2 5 CH c CH 3 CHClCHOHCH 3 d CH 3 CH 2. The curved arrow in structure B represents type 2 resonance motion - the pi bond breaks to form a new pi bond to the carbocation carbon.
Um and so the first molecule that were given a eyes ozone and so ozone has to possible resident structures. GET 20 OFF GRADE YEARLY SUBSCRIPTION. We have some lone pairs resonance structure here.
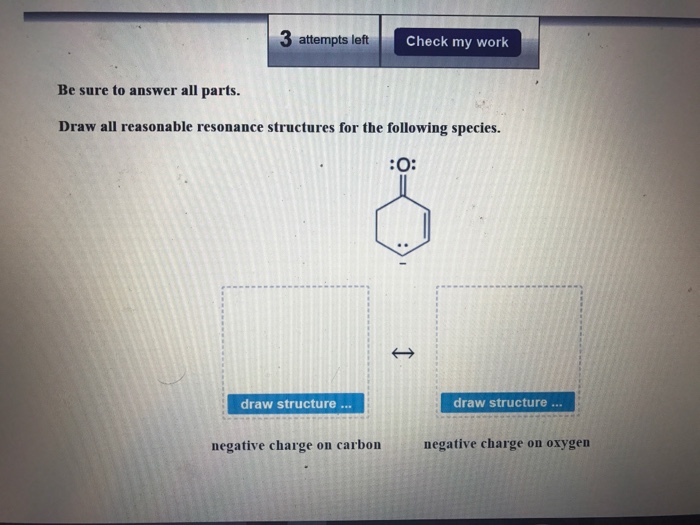
We can obtain the following resonance structures by following the same steps as mentioned above. Draw all reasonable resonance structures for the following species. Then draw the resonance hybrid.
Well the only carbons that are really are Adams RSP to in this are going to be a long this. Be sure to answer all parts. Ah And in this problem were asked to draw all reasonable resident structures for each of these four species that were given.
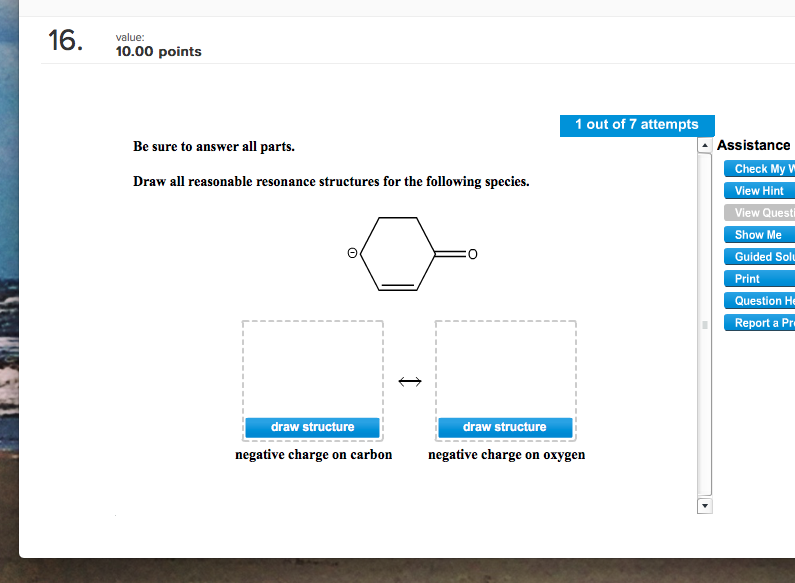
Negative charge on oxygen negative charge on carbon. Draw all reasonable resonance structures for each species. The two resonance structures in this example are non-equivalent so one is more stable than the otherBy applying the formal charge guideline the - formal charge is more preferable on oxygen which is more electronegative than nitrogen so the 2 nd structure is the more stable one with lower energy and makes more contribution to the actual structure in this species.
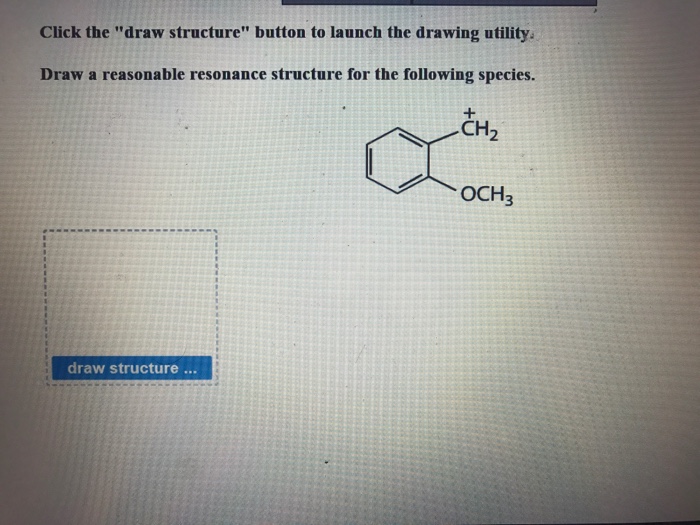
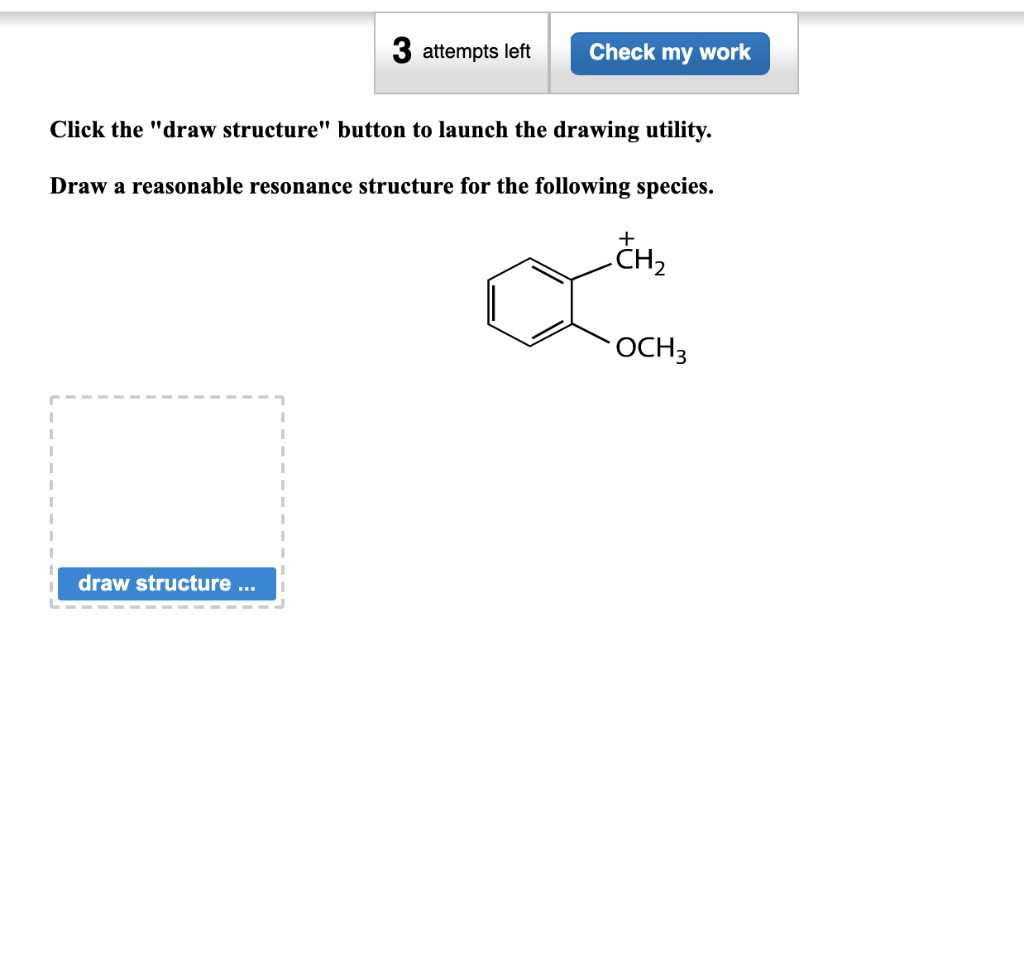
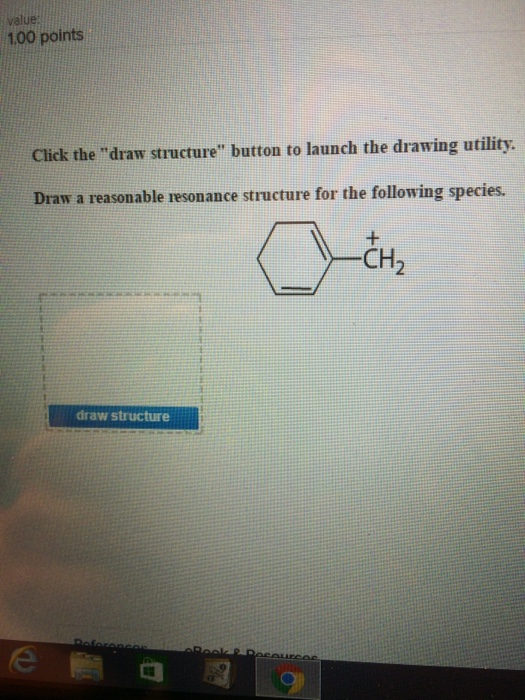
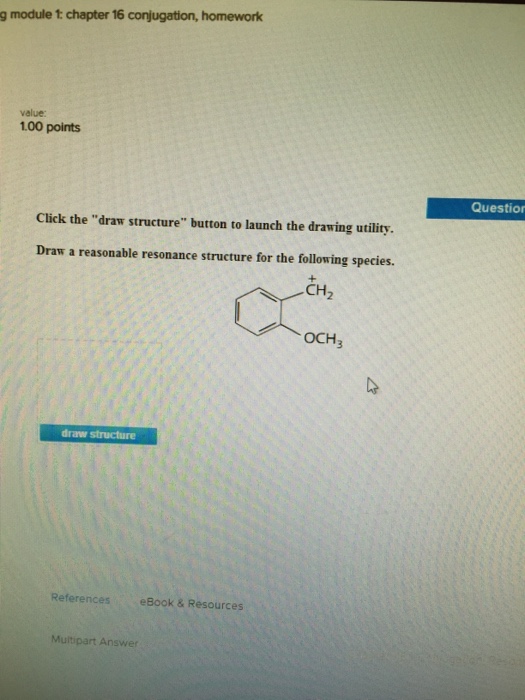
Draw structure draw structure. Draw a reasonable resonance structure for the following species. We review their content and use your feedback to keep the quality high.
If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above the partial charge on each oxygen atom will be equal to - ⅔. The net charge on the central atom remains 1. Determine the number of resonance structures for eachstructure.
To understand this concept say that someone asks you to describe a mermaid. 98 95 ratings Transcribed image text. Mr draws many president structures as we can for each of the following species.
Draw all reasonable resonance structures for the following species. All the only different resident structure is the one indicated. We review their content and use your feedback to keep the quality high.
Be sure to answer all parts. The center 20 appear. So lets go ahead and start with a So now remember for resident structures we can Onley move electrons and we cant create new bonds.
This goes in here. Resonance structure of any species is created by the steporange ment of TT ps bond No Sigma co bond participilate in resonance. And this problem asks us to draw all reasonable residents structures for each molecule that were given in the problem.
But we can further resonate this thie end with this bottom position or resonates to this bond. We review their content and use your feedback to keep the quality high. Likewise you could also say a fish but that too would only be part of the answer.
For each of the following species how many reasonable resonance structures are there including the original structure. Write any non-zero formal charges on the appropriate atoms show all lone pairs of electrons as pairs of dots and all bond pairs as lines. The curved arrow in structure A represents the type 3 resonance motion - the pi bond between the carbon and oxygen breaks to form another lone pair on the oxygen.
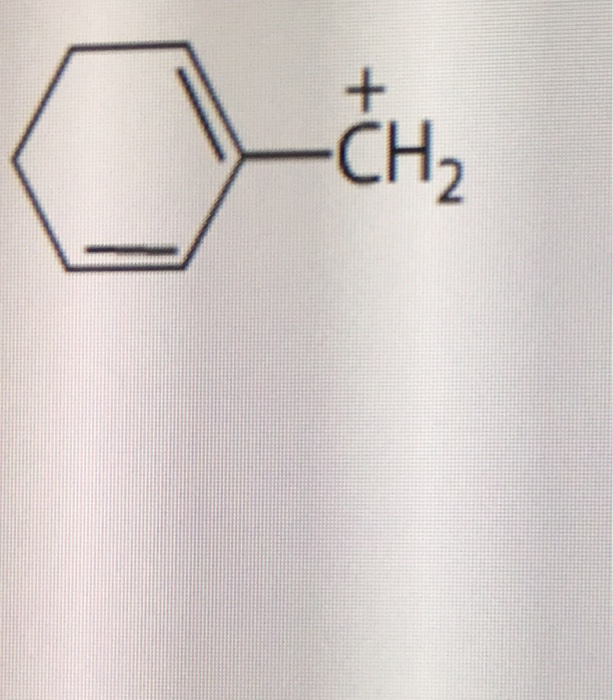
Convert each molecule into a skeletal structure. Draw four additional resonance structures for the following cation. Draw the resonance structures without changing the position of atoms and bonds.
This resonance hybrid is illustrated below. The number of unpaired electrons in each resonance structures should be same. It is more preferable for negative formal charges to be on oxygen the more electronegative atom.
Up to 256 cash back Get the detailed answer. Resonance structure must be a valid Lewis structure. Therefore structure 2 is the most stable one.
CH2 N Upload Choose a File. This is the answer to Chapter one problem number 55 from the Smith Organic Chemistry textbook. Draw the resonance structures by using by using the following three rules they are.
Experts are tested by Chegg as specialists in their subject area. You could say a human but that would only be part of the answer. Problem number 33 Fromthe Smith Organic chemistry textbook.
Previous question Next question. 2 Do not break single bonds. Rules for drawing resonance structures.
Draw in all the carbon and hydrogen atoms in each molecule. For both structures 1 and 2 the formal charge is -1. Step-by-step explanation Image transcriptions Sol.
Chemistry questions and answers. Draw all reasonable resonance structures for the following species. The three possible resonance structures of NO 3 are illustrated below.
Um And so in an effort to cut down on the length of time this video is going to take I went ahead and. Each individual drawing that composes the hybrid is called a resonance structure. Draw all reasonable resonance structures for the following compound.
Experts are tested by Chegg as specialists in their subject area. Therefore we can draw resonance structures for O 3 molecule as follows. So lets see what we can kind of do.
A ClO 3 chlorate - Although the below seven are chemically reasonable Lewis structures ie the upper left one satisfies the octet. View the full answer. Negative charge on carbon negative charge on.
For each of the following species how many reasonable resonance LIMITED TIME OFFER. 5 PAGES 234 - 235 Draw all chemically reasonable resonance structures for the following species. 4 Question Please help me find how many possible resonance structures there are for question d and e.
1 Do not exceed the octet on 2nd-row elements. Oh mhm ch two minus. Draw all of the resonance structures for azide anion N3 and indicate the most stable o ne.
Be sure to answer all parts. This double bond comes out here you would have h 3 cc oh minus double bond ch two for be Gods six member ring. Double bond double bond.
Use curved arrow notation to draw all reasonable resonance structures for the structures shown below. On the right hand side the astute among you will notice that all three of these structures which of jet here here and here are the exact same.
Solved Draw A Reasonable Resonance Structure For The Chegg Com
Solved Click The Draw Structure Button To Launch The Chegg Com
Solved Draw A Reasonable Resonance Structure For The Chegg Com
Solved Draw A Reasonable Resonance Structure For The Chegg Com
Solved Draw A Reasonable Resonance Structure For The Following Species Draw Out All Bonds To The Oxygen Atom In Your Answer Ls Course Hero

Draw A Reasonable Resonance Structure For The Following Species Study Com
Solved Draw All Reasonable Resonance Structures For The Chegg Com
0 comments
Post a Comment